1.uncut sheet for urine test low price Uncut Sheet,Uncut Sheet Strips,Rapid Uncut Sheet,Uncut Sheet Urine Strips Changchun LYZ Technology Co., Ltd , https://www.lyzstrips.com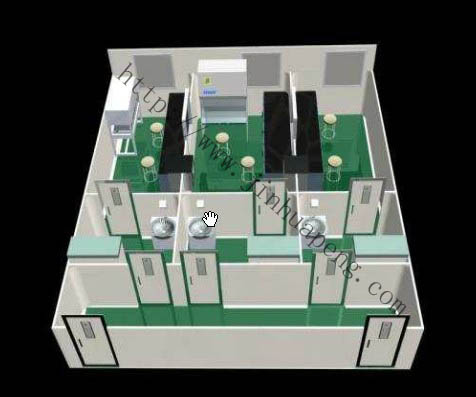

2.uncut sheet for urine test high quality
3.uncut sheet for urine test rapidly
4.uncut sheet for urine test OEM design welcome
The benefit to purchase rapid uncut sheet for urine test strip :
To help you saving the shipping cost
To help you being the manufacture
To help you saving the import tax as the materials
Our factory can supply more than 30 kinds of rapid test sheets
Package: aluminum foil package
Advantage: vacuum package can protect the effect of moisture
PCR laboratory design
1 PCR laboratory floor layout
The PCR amplification test laboratory is in principle divided into four separate work areas: reagent storage and preparation area, sample preparation area, amplification reaction mixture preparation and amplification area, and amplification product analysis area. In order to avoid cross-contamination, entering each working area must strictly follow a single direction, that is, only from the reagent storage and preparation area → specimen preparation area → amplification reaction mixture preparation and amplification area → amplification product analysis area.
Reagent and sample transfer between each experimental zone should be performed through a transfer window. The schematic diagram of the PCR laboratory layout is shown in the figure below.
2 laboratory air conditioning ventilation system design and pressure control
PCR laboratories do not have strict purification requirements, but in order to avoid the possibility of cross-contamination between the various experimental areas, it is advisable to use a full-flow full-flow airflow organization. At the same time, the proportion of sending and exhausting should be strictly controlled to ensure the pressure requirements of each experimental area.
2.1 PCR reagent storage and preparation area
The main operations in this experimental zone are the preparation of the storage reagent, the dispensing of the reagents, and the preparation of the main reaction mixture. Reagents and materials used for sample preparation should be shipped directly to the area and must not pass through other areas. The reagent raw materials must be stored in the area and prepared into the required storage reagents in the area.
There are no strict requirements for the control of airflow pressure in this area.
2.2 PCR specimen preparation area
The main operations in this region are sample storage, nucleic acid (RNA, DNA) extraction, storage and addition to the amplification reaction tube and determination of DNA synthesis.
The pressure gradient requirements for this area are: positive pressure relative to adjacent areas to avoid aerosol contamination from adjacent areas into the area. In addition, due to the possibility of aerosol-induced contamination during the sample loading operation, unnecessary movement in the area should be avoided.
2.3 amplification reaction mixture preparation and amplification zone
The main operation in this region is DNA amplification. Further, the addition of the prepared DNA template (from the sample preparation zone) and the main reaction mixture (from the reagent storage and preparation zone) to a reaction mixture or the like can also be carried out in the zone.
The pressure gradient in this area is required to be negative relative to the adjacent area to avoid aerosol leakage from the area. In order to avoid contamination caused by aerosols, unnecessary movement in the area should be minimized. Individual operations such as loading should be performed in a clean bench.
2.4 amplification product analysis area
The main operation in this region is the determination of amplified fragments.
This area is the most important source of amplification product contamination. Therefore, the pressure gradient in this area is required to be negative pressure relative to the adjacent area to avoid diffusion of amplification products from this area to other areas.
3 pollution prevention and control
The core issue in PCR laboratory design is how to avoid contamination. In practice, the following types of pollution are common: contamination of amplification products; contamination of natural genomic DNA; contamination of reagents and contamination between specimens. Since pollution occurs, the experiment must be stopped until a source of contamination is found, and the results of the experiment must be discarded and the experiment needs to be repeated. Therefore, it is not only time-consuming and cumbersome to waste around the laboratory to find pollution sources after pollution occurs, which wastes manpower and resources. Therefore, to avoid pollution, it should be prevention first, not exclusion.
3.1 Strict division of work area
(1) The setting of each experimental area is reasonable;
(2) Each experimental area should have obvious marks (such as eye-catching house numbers or different ground colors, etc.) to avoid confusion between equipment items and reagents in different experimental areas.
3.2 Reasonable system settings
(1) Reasonable air-conditioning ventilation system setting, try to use the full-send air-conditioning system;
(2) Strict airflow pressure control to ensure different pressure requirements in different experimental zones.
3.3 Specification operation
(1) The technicians of the augmentation testing laboratory must conduct on-the-job training, and they can only engage in clinical gene amplification testing after passing the training;
(2) During the experimental operation, the operator must wear gloves and replace them frequently. In addition, the use of disposable caps in operation is also a measure to effectively prevent contamination;
(3) The cleaning work is timely and correct. The area must be cleaned immediately after the end of the experiment. In addition to the conventional disinfectant liquid to wipe the surface disinfection or UV lamp disinfection, some experimental equipment should also be autoclaved.
3.4 Strict management
(1) Strictly control the personnel entering and leaving the laboratory. Persons unrelated to the experiment shall not enter or leave the laboratory at will, and if necessary, set up independent passages and doors that enter and exit the entire experimental area;
(2) Minimize unnecessary movement in the experimental area to reduce the possibility of cross-contamination.
(3) The amplification product analysis area is the most important source of amplification product contamination. The waste liquid cannot be dumped in the laboratory. It must be immersed in the disinfectant solution and discarded in the place away from the laboratory. The used tips are disposable. The material should also be treated by immersion and disinfection in a disinfectant solution, such as incineration;
(4) Some gene mutations and toxic substances may be used in the amplification product analysis area, and special attention should be paid to the safety protection of the experimental personnel.
3.5 Complete laboratory facilities
Complete laboratory supporting facilities are necessary conditions for ensuring experimental work. Equipment and instruments should be equipped according to the experimental contents of each laboratory, such as ultra-clean workbench, centrifuge, and sampler.